Nuclear
Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI) have been
involved in almost all facets of science ranging from discovering unknowns
within samples to expanding our knowledge of the human body.
 Although grand,
these instruments have their limitations and it is through nitrogen-vacancy
(NV) centers that these limitations will be abolished.
The primary scale in which science can scan at the moment only reaches down to
the micrometer and to go any further involves a large amount of money and time
or specific subzero temperatures2.
Although grand,
these instruments have their limitations and it is through nitrogen-vacancy
(NV) centers that these limitations will be abolished.
The primary scale in which science can scan at the moment only reaches down to
the micrometer and to go any further involves a large amount of money and time
or specific subzero temperatures2.
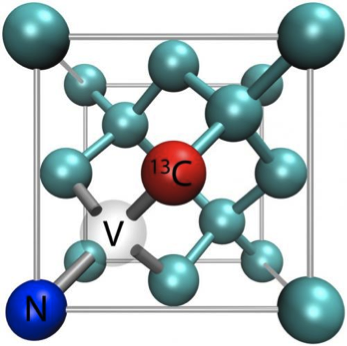
NV centers are able to circumvent
the current expensive and temperature locked methods by not requiring external
magnetic fields and using synthetic diamonds that can gather information at ambient
temperatures1. NV centers are defects found within diamonds which
have been proven to detect proton nuclear spins in samples within a volume of 5
cubic nanometers. When the sample, atop the diamond detector, is subjected to
radio waves there is a fluorescent response measuring excitation and relaxation
spins of the protons within the sample that is transformed into interpretable
data by computers3.
Although
different from standard NMR and MRI instrumentation the use of magnetic
resonance is the same. Imaging produced through the use of NV centers is still
in the works as MRI is a form of NMR so too will its advance come once NMR is
mastered under these new conditions.
Monday, May 12, 2014
Written by Jason Bingaman
Sources
1.
Kemsley, J. “Taking NMR And MRI To The Nanoscale”, Chemical
and Engineering News, Vol. 91 Issue 5 Pg. 4, February 4, 2013. https://cen.acs.org/articles/91/i51/Taking-NMR-MRI-Nanoscale.html
2.
Reinhard, F. et al. “Nuclear Magnetic Resonance Spectroscopy
on a (5-Nanometer)3 Sample Volume”. http://www.sciencemag.org/content/339/6119/561
3.
Rugar, D. et al. “Nanoscale Nuclear Magnetic Resonance with
a Nitrogen-Vacancy Spin Sensor”. http://www.sciencemag.org/content/339/6119/557
Cyanate
esters are a remarkable family of compounds that exhibit many useful properties
in the materials world. They retain their structural integrity into the 400⁰ C
range, they have extremely low water uptake, making them ideal for
corrosion-resistant coatings which the Navy requires for its sea-borne assets.
At
China Lake we set out to create a compound with these properties that would
make an attractive replacement for the current standard which is a primer laced
with Chromium (a very toxic carcinogen). The structure shown above is the final
product of our work which stemmed from an initial reaction using carvacrol.
Carvacrol was attractive because of its structural similarity to carvone,
limonene, and pinene. These chemicals, as their names imply come from and
attribute to the aromas associated with caraway, citrus, and pine trees
respectively. They are often the waste products of the industries that
This is a Thermal Gravimetric Analysis (TGA) which shows our
product’s high durability under intense heat. It retains almost all of its mass
up until the 400⁰ C mark as is common for molecules of this family.
After sending our final product off for characterization, we set to work on finding a way to take one or all of these precursor molecules to complete synthesis. Using a series of small scale reactions, we were able to go from limonene to the final product with relative ease, thus showing the viability of this as a cheap and less toxic alternative coating methods. Current issues regarding our experiment center around high enough yields to be viable and taking pinene all the way to our finished compound. We were able to get small yields that would potentially work, but they were clouded by other isomers mixed in so a new reaction will need to be tried in order to get a more selective result.
Future Work
Now that the as of yet unnamed molecule has been characterized,
we will continue to refine the overall reaction process to improve yield as
well as find ways to selectively react our precursor molecules. From there our
final challenge will be scaling the reaction up in size to accommodate for
large scale production.
Written by Thomas Koontz