The disappearance of Xenon, thought to be present in abundance on early earth, has baffled scientists for decades. Now new evidence backs up a theory that Xenon has displaced the silicon in quartz (SiO2) and has been literally under our noses in the earths crust.
In the 1970s scientists noticed a mysterious lack of Xenon in the earths atmosphere and mantle. Estimates suggested that upwards of 90% of the Xenon thought to be on early earth had vanished seemingly without a trace. Some speculated that the Xenon escaped into space or was frozen into ice caps; however, the plentiful presence of all of the other noble gases threw a wrench in many of these early theories.
Though Xenon is a noble gas, typically inert by nature, it is the most reactive of all the noble gases. In 2005 it was proposed that under high-temperature and high-pressure conditions it would be possible for Xenon to displace Silicon in SiO2. While the theory explained the lack of Xenon in the earth’s atmosphere and mantle, a new mystery arose: Prior to now XeO3 and XeO4 were the only known Xenon Oxides; where was the Xenon Dioxide (XeO2)?
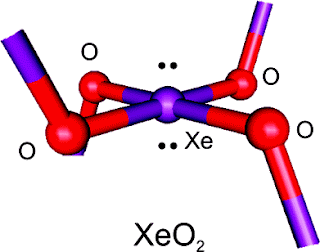
A team of Canadian researchers provided this missing link to the Silicon displacement theory with results from the first ever synthesis and spectroscopic characterization of XeO2 published in the 22nd of February issue of the Journal of the American Chemical Society. The researchers found XeO2 to have an extended network in which xenon is linked to four neighboring oxygen atoms in a XeO4 square-planar geometry.
Since scientists are being made to chuck their previous assumption that noble gases are always going to behave in an inert fashion, these findings may change the way geochemists do business altogether.
In the 1970s scientists noticed a mysterious lack of Xenon in the earths atmosphere and mantle. Estimates suggested that upwards of 90% of the Xenon thought to be on early earth had vanished seemingly without a trace. Some speculated that the Xenon escaped into space or was frozen into ice caps; however, the plentiful presence of all of the other noble gases threw a wrench in many of these early theories.
Though Xenon is a noble gas, typically inert by nature, it is the most reactive of all the noble gases. In 2005 it was proposed that under high-temperature and high-pressure conditions it would be possible for Xenon to displace Silicon in SiO2. While the theory explained the lack of Xenon in the earth’s atmosphere and mantle, a new mystery arose: Prior to now XeO3 and XeO4 were the only known Xenon Oxides; where was the Xenon Dioxide (XeO2)?
A team of Canadian researchers provided this missing link to the Silicon displacement theory with results from the first ever synthesis and spectroscopic characterization of XeO2 published in the 22nd of February issue of the Journal of the American Chemical Society. The researchers found XeO2 to have an extended network in which xenon is linked to four neighboring oxygen atoms in a XeO4 square-planar geometry.
Since scientists are being made to chuck their previous assumption that noble gases are always going to behave in an inert fashion, these findings may change the way geochemists do business altogether.

Post a Comment